 Nano Aerosol Chamber In-Vitro Toxicity
Nano Aerosol Chamber In-Vitro Toxicity
Effects of inhaled nanoparticles to normal and diseased airway epithelia by in vitro technology
Inhalation of engineered nanoparticles handled as powders, dispersions or sprays in industrial processes and in consumer products pose a potential and largely unknown risk for incidental exposure. Thereby individuals with chronic lung diseases, as well as children and the elderly are expected to be more vulnerable to adverse effects of inhaled engineered nanoparticles than adult healthy subjects.
Introduction
It is the aim of this research to investigate health impacts of widely used engineered nanoparticles (ENP) upon inhalation by comparing normal and susceptible, i.e., diseased, lungs using in vitro technology.
We focus on healthy adults and adult patients with prevalent chronic respiratory diseases, i.e., chronic obstructive pulmonary disease (COPD, smokers), allergic asthma (house dust mites) and cystic fibrosis (CF). |
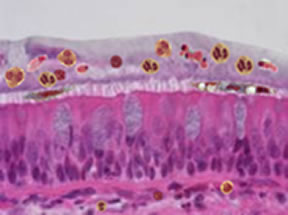 Schematic of inner lung surface in disease |
Analysis of nanoparticle toxicity is performed in primary human airway epithelial cells (HBEC), i.e., fully differentiated air-liquid interface (ALI) airway epithelia. This tissue is the immediate target of inhaled particles and known to play a major role in lung disease.
Cells are exposed in NACIVT, the broadly applicable nano aerosol deposition chamber for efficient and quantitative deposition of nanoparticles, out of a continuous air-stream, thus reflecting the in-vivo situation. The chamber is transportable and mobile to be used at any particle source of interest and there is no limitation with respect to the kind of nanoparticles or fibers used.
Schematic of NACIVT, the Nano Aerosol Deposition Chamber for In Vitro toxicity
The toxicity of current commercial ENP, which have a high risk to be inhaled are tested, i.e., titanium dioxide, carbon black and silver as well as consumer products containing ENP i.e., deodorant or impregnation sprays. Dose-response curves are established in HBEC from normal and diseased donors. Additionally, the influence of nanoparticle aggregation is assessed.
Interaction of particles with the organism: Routes of entry, barriers
The organism is delimited by barriers from the environment, but also allows "cross-border traffic".
Test System NACIVT: the Nano Aerosol Deposition Chamber for In Vitro Toxicity
The research team uses the portable and realistic test system with cell cultures which replicate the inner lung surface. The versatile system can be used for particles from various particle sources and for a variety of cell cultures, allowing realistic in vitro toxicity testing.
Circumferential view of NACIVT.
Chamber Characterization
The research team characterized NACIVT regarding its performance and investigates the effects of inhaled nanoparticles to normal and diseased lung tissue.
Applications
Nanoparticles in powders, dispersions or sprays pose a yet unknown risk for incidental exposure, especially in persons with lung disease, children and the elderly. Safety testing of abundant engineered nanoparticles (ENP) is performed in cells from normal and diseased lungs using the novel, portable and realistic nano aerosol deposition chamber NACIVT.
Publications
The findings of the studies conducted by the research team can be found in selected publications.